Camila Harumi Kimura
Advisor: Profa. Dra. Mari Cleide Sogayar
Supervisor: Isaura Beatriz Borges Silva
Undergraduate biomedicine student at the Institute of Biomedical Sciences of the University of São Paulo (ICB – USP). Develops a Scientific Initiation project in regenerative medicine for type 1 diabetes mellitus, using peptide growth/differentiation factors in the differentiation process of murine embryonic stem cells (mESCs) into insulin-producing cells (IPCs).
The diabetes mellitus (DM) is a metabolic disease characterized by either the complete deficiency in insulin production (type 1 diabetes mellitus – DM1) or the organism’s inability to properly use this hormone (type 2 diabetes mellitus – DM2), both leading to increased blood sugar (glucose) levels. Hyperglycemia is the main condition presented by patients with DM and, if not controlled, can cause chronic complications, such as nephropathy, cardiovascular diseases (acute myocardial infarction, stroke, and peripheral arterial disease), retinopathy, and diabetic neuropathy.
Insulin therapy, the main form of treatment for patients with DM1, in most cases, is unable to maintain perfect control of glycemic levels (Diabetes Control and Complications Trials Research Group – DCCT, 1993). Physiologically, restoration of pancreatic β-cell functions through transplantation of the insulin-producing tissue (whole pancreas or pancreatic islets) may be the best therapeutic option. However, whole organ pancreas transplantation is a major surgical intervention, which requires a very strict immunosuppressive regimen, recommended only for patients who need a kidney transplant and depend on the proper functioning of the pancreas for successful treatment (Leal-Lopes, 2018). Thus, the transplantation of pancreatic islets, introduced in Brazil by our research group (Eliaschewitz et al., 2004; 2009), proved to be an interesting alternative to whole pancreas transplantation, considering it is a simpler and
less invasive procedure. Nevertheless, the low availability of pancreas donors and the
difficulty in the maintenance and survival of islets in culture after isolation are limiting points to islet transplantation.
Given the need to prospect new therapeutic interventions for the treatment of DM1, several studies have sought methodologies that allow the expansion of β-cells in vitro and enable the increase in the supply of insulin-producing cells (IPCs – Insulin-Producing Cells) for pancreatic islet replacement therapy. A promising approach to overcome dependence on human pancreatic β-cells for pancreatic islet transplantation is the differentiation of stem cells into insulin-producing cells (Lojudice; Sogayar, 2008).
Different strategies for obtaining insulin-producing β-cells have been adopted by several
research groups worldwide. The NUCEL group has sought to establish a differentiation protocol capable of generating functional insulin-producing cells and in large numbers
(Lojudice, 2008; Kossugue, 2013; Leal-Lopes, 2018). Therefore, this project aims, based on the protocol established by our group, to optimize the process of differentiation of murine embryonic stem cells (mESC) into insulin-producing cells (IPCs) for the treatment of DM1. For this, the peptide growth/differentiation factors BMP-7 (Bone Morphogenetic Protein 7) and PDGF-BB (Platelet-Derived Growth Factor-BB) produced in our laboratory will be used. The interest in employing these factors and evaluating their effects during the in vitro differentiation process is based on the mitogenic potential of PDGF-BB (CHEN et al., 2011) and the ability of BMP-7 to induce differentiation of non-endocrine cells into endocrine cells (KLEIN et al., 2015; QADIR et al., 2018).
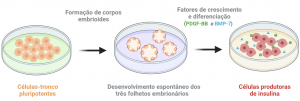
Figura 1: Diferenciação de células-tronco pluripotentes em células produtoras de insulina através da aplicação de fatores de crescimento e diferenciação que participam da organogênese pancreática.
Referências:
Chen, H; Gu, X; Liu, Y; Wang, J; Wirt, SE; Bottino, R; Schorle, H; Sage, J; Kim, SK. PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature. 2011 Oct 12;478(7369):349-55.
Eliaschewitz, FG; Aita, CA; Genzini, T; Noronha, IL; Lojudice, FH; Labriola, L; Krogh, K; Oliveira, EM; Silva, IC; Mendonça, Z; Franco, D; Miranda, MP; Noda, E; de Castro, LA; Andreolli, M; Goldberg, AC; Sogayar, MC. First Brazilian pancreatic islet transplantation in a patient with type 1 diabetes mellitus. Transplant Proc. 2004 May; 36(4): 1117-8. 2004.
Eliaschewitz, FG; Franco, DR; Mares-Guia, TR; Noronha, IL; Labriola, L; Sogayar, MC. Islet transplantation as a clinical tool: present state and future perspectives. Arq Bras Endocrinol Metabol. 2009 Feb; 53(1): 15-23. Review. Portuguese. 2009.
Klein, D; Álvarez-Cubela, S; Lanzoni, G et al., 2015. BMP-7 induces adult human pancreatic exocrine-to-Endocrine conversion. Diabetes 64, 4123–4134.
Kossugue, P. M. Diferenciação de células-tronco embrionárias murinas (mESCs) em células produtoras de insulina (IPCs) e caracterização funcional do gene Purkinje Cell Protein 4 (Pcp4) neste processo. 142f. Tese de Doutorado – Instituto de Química, Universidade de São Paulo, mai. 2013.
Leal-Lopes, C. Terapias alternativas para o Diabetes Mellitus tipo 1: caracterização funcional do gene Txnip na diferenciação β-pancreática e desenvolvimento de biomaterial inovador para microencapsulamento celular. 203f. Tese de Doutorado – Instituto de Química Universidade de São Paulo, jun. 2018.
Lojudice, FH. Identificação de genes diferencialmente expressos durante diferenciação de células-tronco e caracterização de células progenitoras mesenquimais. 173f. Tese de Doutorado em Ciências Biológicas (Bioquímica) – Instituto de Química, Universidade de São Paulo, 2008.
Lojudice, FH; Sogayar, MC. Células-tronco no tratamento e cura do diabetes mellitus. Ciência & Saúde Coletiva, v. 13, n. 1, p. 19-21, 2008.
Qadir, MMF; Álvarez-Cubela, S; Klein, D et al., 2018. P2RY1/ALK3-expressing cells within the adult human exocrine pancreas are BMP-7 expandable and exhibit progenitor-like characteristics. Cell Rep. 22, 2455–2468.
The Diabetes Control and Complications Trials (DCCT) Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes, v. 46, n. 2, p. 271-286, fev. 1997.

